 |
 |
 |
 |
 |
 |
|||||||
|
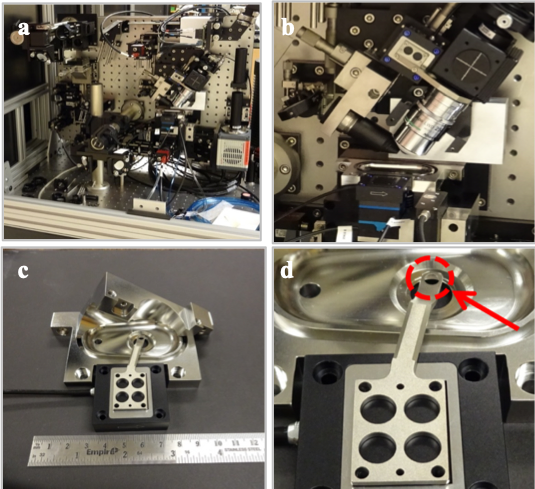
Robert Anderson — NanoScience and NanoEngineering The optical spectroscopy laboratory, room 245, is a 450 sq. ft. laser laboratory with chilled water drops, hepa-filtered air handlers and supporting instrumentation. 3 main optical tables housing high powered lasers, including a high repetition rate (40MHz), high energy (1 μJ) 1064nm mode locked laser (Time-Bandwidth Fortiss™), low rep-rate (10 Hz) high energy (1 mJ) cavity dumped Nd:YAG laser system (Continuum Leopard™), time correlated single photon counting apparatus and a custom-built Lattice Light Sheet Microscope as described described below. Lattice Light Sheet Microscope The custom-built lattice light-sheet microscope (LLSM), constructed through a license agreement with the Howard Hughes Medical Institute (HHMI). The lattice light-sheet microscope (Figure 1) is a refinement on the idea of selective-plane illumination microscopy (SPIM), where sample excitation is a thin sheet of light, sectioning the sample and reducing background fluorescence, photo-bleaching, and photo-toxicity. Sampling frame rates are dramatically improved by widefield imaging of the illuminated plane with the detection objective oriented normal to the excitation plane (Figure 1a, 1b). The sample chamber (Figure 1c) is a water immersion bath with three water immersion objectives (excitation, detection and epi-illumination) and two possible observation beam-paths (light-sheet detection objective and epi-illumination objective) through an aperture in the bottom of the sample chamber. Sample positioning is accomplished via a piezo-actuated translation stage of a custom designed sample holder (figure 1c, 1d) capable of submicron resolution. Detection on the LLSM is performed with two high-speed Hamamatsu Orca Flash 4.0 v2 CMOS cameras capable of a full 100 frames per second at a pixel count of 2048x2048 and grayscale resolution of 16 bits, producing 800 mega-bytes of data per second per camera. Data capture and post-processing are provided by a rack-mounted instrument control server and high-speed disk array located at SDSM&T. The microscope is equipped with a laser combiner comprising seven wavelengths: 642nm, 589nm, 560nm, 532nm, 488nm, 445nm and 405nm. This allows the excitation of a wide variety of fluorescent tags. The current software allows up to six wavelengths to be utilized in a single imaging scan by multiplexing in time.
Lattice Light Sheet Microscopy Image Gallery Recent lattice light sheet microscopy imagery, click on the image to view the animation (hyperlinked) ... (Credit Brandon Scott and Robert Anderson). 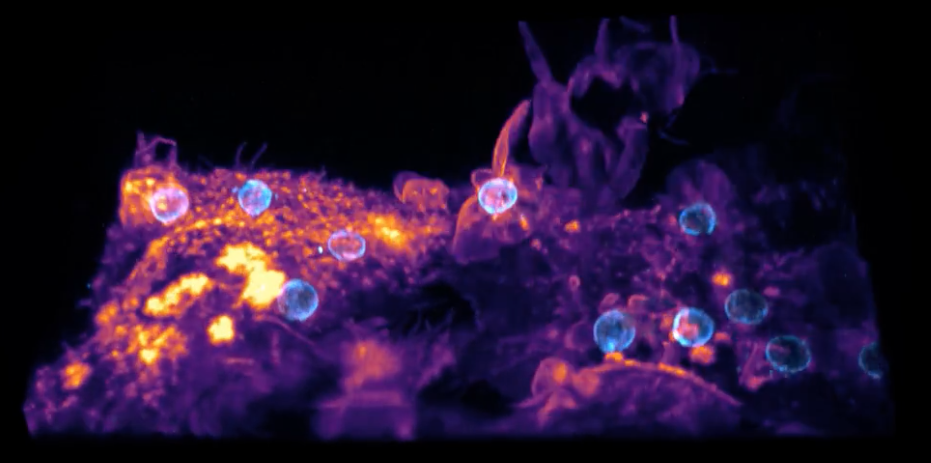    
| ||||||||||||